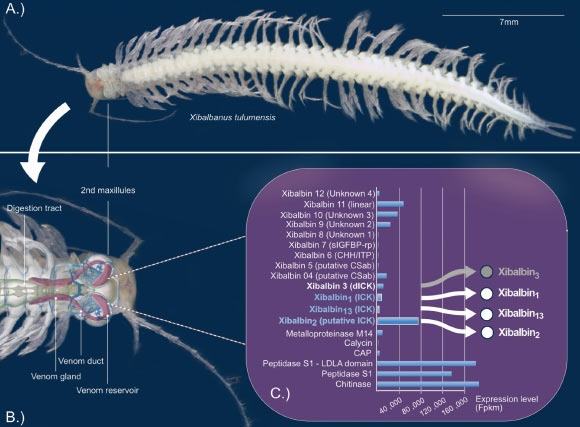
Xibalbanus tulumensis, a venomous remipede came upon in anchialine caves on the Yucatán Peninsula, is the most productive crustacean for which a venom system has been described.
Xibalbanus tulumensis. Image credit rating: Pinheiro-Junior et al., doi: 10.1186/s12915-024-01955-5.
“Venomous animals inject their toxic compounds into different organisms essentially for self-defense or predation,” stated Dr. Björn von Reumont, a researcher at Goethe University Frankfurt, and his colleagues.
“Tons of venoms comprise proteins that hold evolved to modulate a fluctuate of physiological capabilities in their intention organisms.”
“Investigating these bioactivities could perchance well simply lead to pharmacological or agrochemical capabilities.”
“The majority of venoms and venom proteins that hold been thoroughly studied mainly accept as true with from iconic and terrestrial groups similar to snakes, spiders, scorpions, and insects,” they stated.
“Marine species hold bought little be taught consideration, with easiest a little assortment of fish and invertebrate species — similar to sea anemones, jellyfish, cone snails, cephalopods, polychaetes, and no longer too long ago nemerteans — being better studied.”
“As venoms and their toxic proteins hold independently evolved in numerous animal lineages, researching unique lineages presents on the one hand but some other to name fresh venom compounds with bright bioactivity and on the different hand to make stronger our working out of the evolution of convergent functional traits on the total.”
In their watch, the researchers investigated the bioactivity of peptides came upon in venom of the crustacean species Xibalbanus tulumensis.
This underwater cave-predicament crustacean belongs to the class Remipedia, which became once first described in the Eighties and at this time contains 28 living species.
“Xibalbanus tulumensis lives in the cenotes which could well perchance well be the underwater cave programs on the Mexican Yucatan peninsula,” the scientists stated.
“The cave dweller injects the venom produced in its venom gland at once into its prey.”
“This toxin accommodates an excellent deal of ingredients, including a novel model of peptide, named xibalbine, after its crustacean producer.”
“Nearly all these xibalbines have a characteristic structural ingredient that’s acquainted from different toxins, especially those produced by spiders: several amino acids (cysteines) of the peptide are certain to each different in this kind of system that they create a knot-like building.”
“This in turn makes the peptides immune to enzymes, heat and shameful pH values.”
“Such knottins most frequently act as neurotoxins, interacting with ion channels and paralyzing prey — an pause that has furthermore been proposed for some xibalbines.”
The watch reveals that each one the xibalbine peptides tested by the crew — and in particular Xib1, Xib2 and Xib13 — successfully inhibit potassium channels in mammalian programs.
“This inhibition is an excellent deal well-known by creating capsules for a fluctuate of neurological ailments, including epilepsy,” Dr. von Reumont stated.
“Xib1 and Xib13 furthermore demonstrate the facility to inhibit voltage-gated sodium channels, similar to those came upon in nerve or heart muscle cells.”
“To boot to, in the sensory neurons of higher mammals, the 2 peptides can activate two proteins — kinases PKA-II and ERK1/2 — desirous about label transduction.”
“The latter means that they are desirous about misfortune sensitization, which opens up unique approaches in misfortune treatment.”
The crew’s findings were printed in the journal BMC Biology.
_____
E.L. Pinheiro-Junior et al. 2024. Diversely evolved xibalbin variants from remipede venom inhibit potassium channels and activate PKA-II and Erk1/2 signaling. BMC Biol 22, 164; doi: 10.1186/s12915-024-01955-5